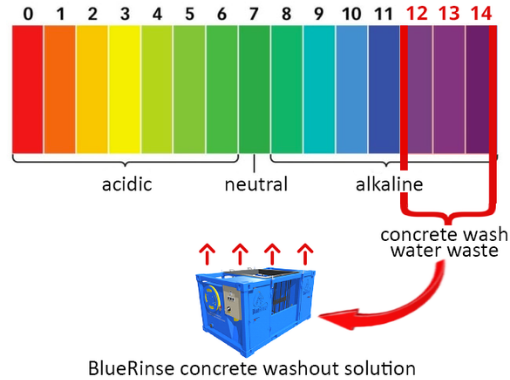
The chemical reaction which causes fresh concrete to slowly stiffen and harden is quite complicated. A by-product of this process is the creation of a highly alkaline chemical that has a natural pH in excess of 12. Drinking water, by comparison, has a pH of around 6.5 to 7.
A pH limit of between 6 and 9 usually won’t cause any damage when released into the environment, but the release of untreated concrete wash water into surface or ground water can have catastrophic effects.
To make high alkalinity wash water safe would normally require dilution on a massive scale. For example, 100,000 litres of clean water would be required to effectively dilute one litre of pH12 concrete wash water. If this is not done correctly, it could exacerbate the problem on a massive scale.
Our BlueRinse Concrete Washout System has been developed to help workers on all sizes of construction sites treat, recycle and re-use their wash water to avoid hazardous storage and costly accumulation.
Visit our Products page to see our full range of solutions, designed to offer peace of mind, protect the environment and suit your individual site policies/regulations.